Micropores pave the way for infection research
_1763369329.JPG)
A new technology allows to create more realistic organ-on-chip environments by allowing a better transport of cells and particles that simulate microorganisms.
A new study provides a powerful way to study infections in environments that closely mimic human organs. The strategy, tested in a bone-marrow-on-chip model, was developed by researchers from the Barcelona Institute for Global Health (ISGlobal), the Germans Trias i Pujol Research Institute (IGTP), the Instituto de Investigaçao e Innovaçao em Saúde (I3S) at Porto University, and the Institute of Nanoscience and Nanotechnology of the University of Barcelona (IN2UB), as part of the HIDDENVIVAX project, funded by "la Caixa" Foundation.
Some infectious diseases like malaria, leishmaniasis, or HIV, establish chronic, latent infections in places of the body that are very difficult to reach and study, such as the bone marrow. These hidden reservoirs allow the pathogen to evade immune responses and drug treatments. They also make it very hard for scientists to observe what happens directly in patients, both for technical and ethical reasons.
To overcome this challenge, researchers are increasingly using "organ-on-a-chip" technology: tiny devices in which human cells are grown to mimic the structure and behaviour of human organs in the lab. These chips often contain gels that imitate the 3D environment of our tissues. "However, many of these gels are too dense, hindering the passage of microbes and immune cells, and movement is essential to recreate how infections really develop," says ICREA researcher Hernando del Portillo, head of the Plasmodium vivax and Exosome (PvREX) Group, who led the study together with Aurora Hernández-Machado from the Condensed Matter Physics at the University of Barcelona and Cristina Barrias from the Bioengineered 3D Microenvironments at the I3S Institute at Porto University.
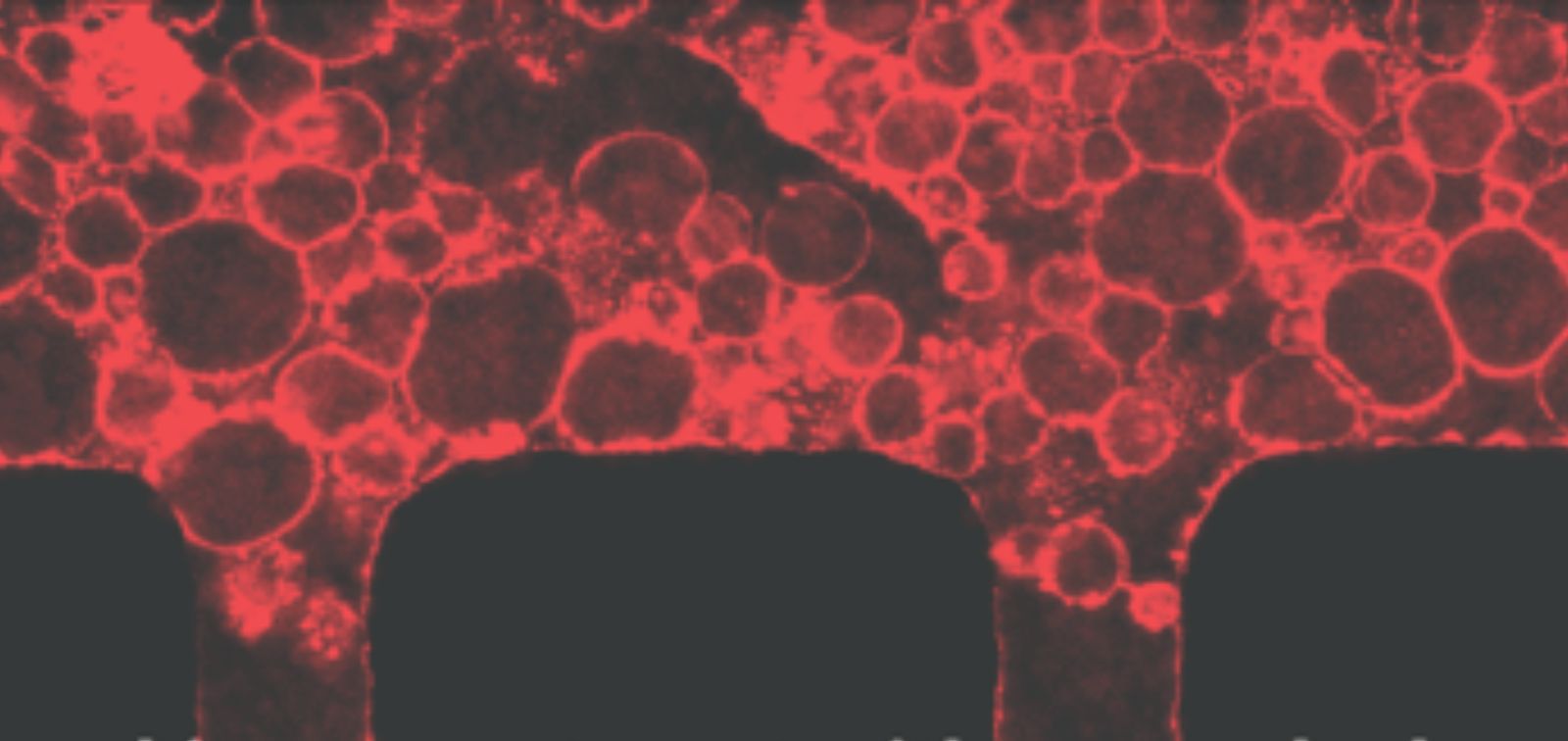
Improved growth, organisation and movement
In this study, the research team developed a new type of porous gel that solves this problem. They mixed small, removable particles of alginate into the gel; once the gel set, they dissolved these particles, leaving behind a network of tiny, interconnected pores. "This makes it much easier for cells - and microorganisms - to move through the gel," says co-author Carmen Fernandez-Becerra, researcher at ISGlobal and IGTP. When tested in a bone-marrow-on-a-chip model, the new material supported better growth and organisation of the different types of bone marrow cells. It also helped the formation of structures similar to blood vessels, allowing fluids and particles that simulate microorganisms to flow more efficiently.
"The alginate microgels used here provide a robust and reproducible way of engineering systems that better simulate infection processes," says Hernando del Portillo. Importantly, these systems should also help to reduce the need for animal studies.
Because this strategy can be applied to different kinds of organ-on-chip systems, it offers a powerful new tool to establish disease models, study host-pathogen interactions and explore therapeutic interventions.
Reference
Caires HR, Fernández-Castillo O, Sima N et al. Advancing Organ-on-Chip Models With a Sacrificial Granular Hydrogel Strategy for Enhanced Permeability and Biomimicry. Small Methods 2025, e00652. DOI: 10.1002/smtd.202500652
