The autonomous ventilation device (DAR) developed by the Hospital Clínic Barcelona, Germans Trias i Pujol, the University of Barcelona and GASN2 is authorized by the AEMPS to start clinical trials
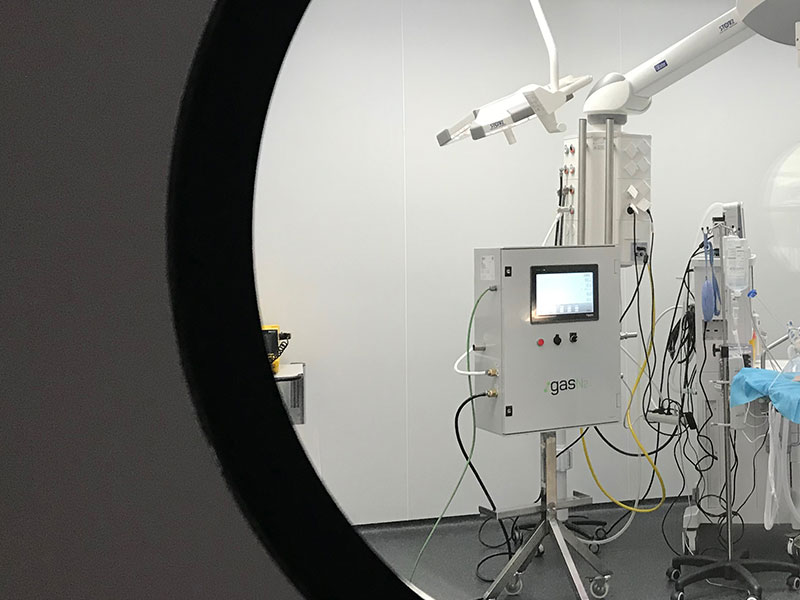
- The Spanish Agency of Medicines and Medical Devices (AEMPS) has today given the green light to test the device developed by the company GasN2. with the support of professionals from the Hospital Clínic Barcelona, the Germans Trias i Pujol Research Institute (IGTP) and the University of Barcelona (UB).
- The prototype uses a system of electro pneumatic valves to supply and control the volume of oxygen necessary for each patient.
- Support from civil society, companies and administrations has been key to the development of the design and industrial scaling up of the model to contribute to the provision of the ventilators needed by the health system.
The Spanish Agency of Medicines and Medical Devices (AEMPS) has authorized a clinical study with the autonomous ventilation device (DAR are it's Spanish inititials) an invasive mechanical ventilator for patients with COVID-19. It is the third device to be developed with the support of professionals from the Hospital Clínic Barcelona, the Germans Trias i Pujol Research Institute (IGTP) and the Faculty of Health Sciences at the University of Barcelona (UB) and to receive authorization.
This clinical trial will be carried out initially at the Hospital Clínic Barcelona and at Can Ruti and will be expanded to other hospitals in Catalonia and the rest of Spain when data is available from the first patients.
This initiative has been made possible thanks to the support received from a large number of private individuals, companies and entities from civil society. It has also received the support of the Catalan Health Service which has brought about the inclusion of companies and has provided support to the centres looking to provide these solutions.
The third device to be certified to fight COVID-19 from these centres
The new equipment uses parts of industrial origin: pneumatic, instrumental, electric and electronic components along with software to control variables during the procedure, monitor screens and visual and audio alarms. It is slightly more sophisticated than the previous two devices that have already been authorized for clinical trials. The DAR allows for the constant objective control of respiratory frequency and other parameters, providing the patient with vital support that they would otherwise not be able to receive, always in the case that a conventional ventilator is not available.
The team supervising the simulations consists of Dr. Josep M. Nicolás, specialist in intensive care at the Hospital Clinic Barcelona and professor at the UB and Dr Ramón Farré, Professor of Physiology at the UB and leader of the group in Respiratory Biophysics and Bioengineering at the IDIBAPs and Dr Manel Puig of the Germans Trias i Pujol Research Institute (IGTP).
The production team is preparing to start manufacture as soon as the AEMPS certifies the device. GASN2 calculate that at first, they can produce 100 machines a day, which could be increased if orders extend to other countries.
