Plasmodium vivax, a Human Malaria Parasite Resilient to Elimination
Why is it so difficult to eliminate the "forgotten" malaria parasite Plasmodium vivax? We present recent scientific data.
[This text has been written by Carmen Fernández Becerra, Associate Research Professor at ISGlobal and Germans Trias i Pujol Research Institute (IGTP) and Hernando del Portillo, ICREA Research Professor]
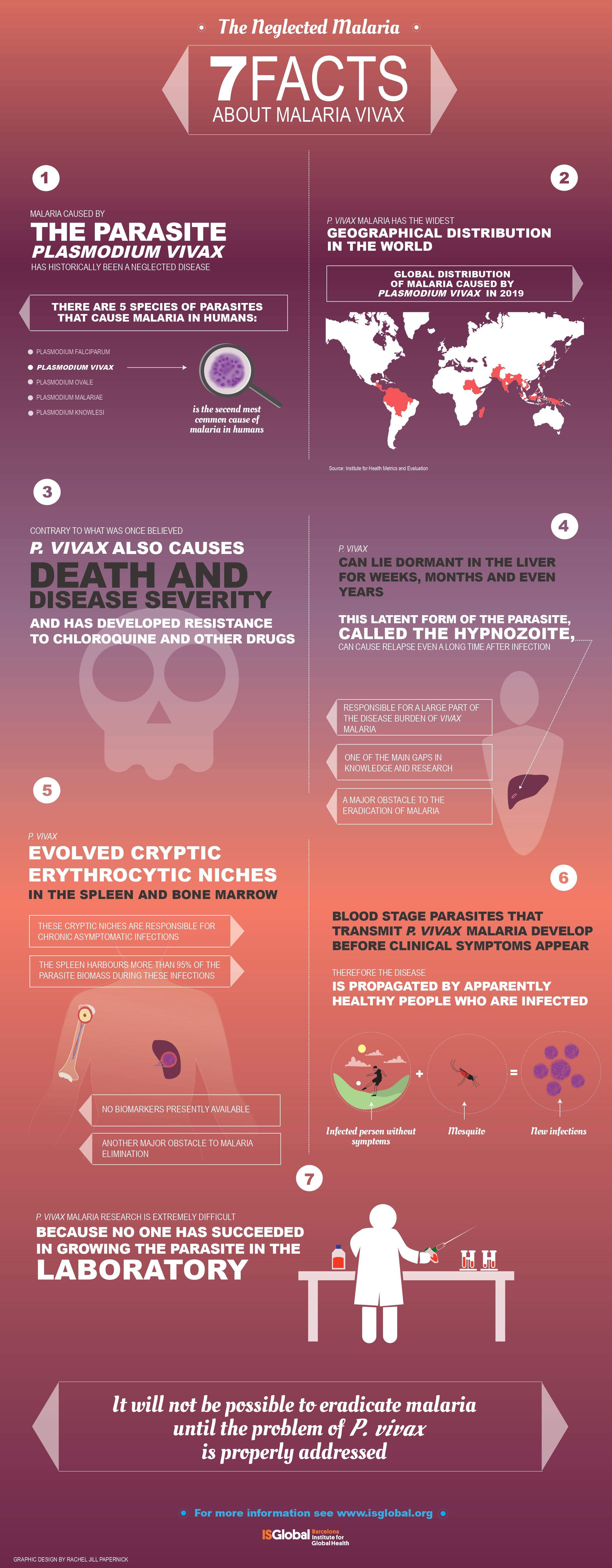
Imagine a world without malaria, a human disease that causes millions of clinical cases a year, more than half million deaths mostly in children under five years old and 2.3 billion people at risk. For centuries, this goal has concentrated in eliminating Plasmodium falciparum, the deadliest malaria species mostly present in sub-Saharan Africa. However, outside this Continent, another species, Plasmodium vivax, is the most widely distributed species. Here, in the World Malaria Day, we highlight recent scientific data that indicates the difficulties in eliminating this neglected parasite.
The spleen, a niche where malaria parasites hide
The spleen is an intriguing organ since ancient times and it is the only organ in our body that filters our blood removing old, deficient and malaria-infected red blood cells. In the particular case of malaria, for centuries it has been considered that the only function of the spleen is destroying parasites. However, this unique function has recently been challenged as a large biomass of intact parasites have been observed in this organ.
Some years ago, our group described the first clear evidences of the presence of a huge parasite biomass in the human spleen. After falling down from a tree in the Brazilian Amazon rain forest, a 19 years old boy experienced an intense abdominal pain and was taken to a Hospital in Manaus where his spleen had to be surgically removed. Unexpectedly, one day after removal of the organ, a diagnostic test made in his blood revealed that at the time he was playing in the tree, he had an infection with malaria. Based on this observation, the removed spleen was examined for the presence of parasites and strikingly it was found to be massively infected by malaria parasites. This first clinical case of an apparently healthy individual indicated that the spleen could represent a niche where malaria parasites could hide during chronic asymptomatic infections. For six years after this first clinical case was reported, spleens from chronic asymptomatic carriers in Timika, Papua, having spleen ruptures and splenectomies were collected and examined. Strikingly in 21/22 spleens malaria parasites were detected. These results thus strongly indicate that P. vivax hides in this organ during chronic asymptomatic infections.
In addition to the spleen, other studies have unequivocally shown that P. vivax can also create cryptic niches in the bone marrow. Of interest, gametocytes, the parasite stage transmitted to the mosquito is readily observed in this tissue, thus indicating a constant sub-microscopy release of gametocytes perpetuating infections caused by this species.
Plasmodium vivax malaria and unique biological features
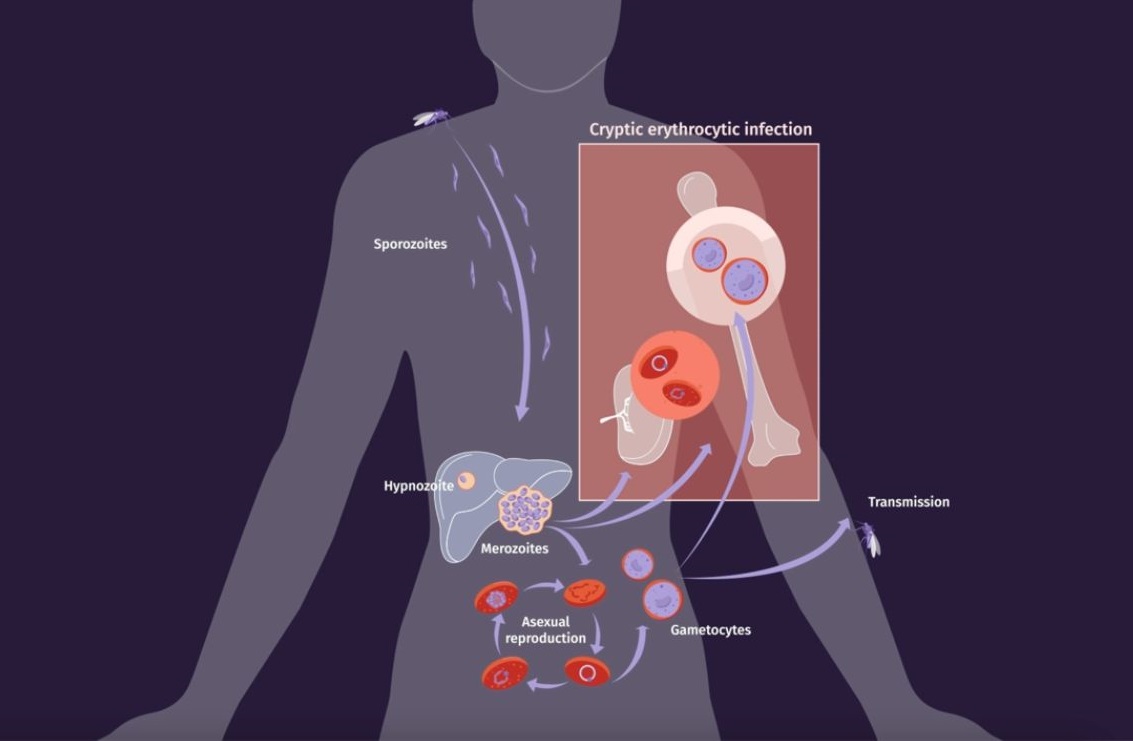
The life cycle of human malaria parasites is very complex. During a mosquito blood meal, female malaria-infected Anopheles mosquitoes inject sporozoites into the bloodstream, initiating the pre-erythrocytic cycle by entering hepatocytes. Within the liver, sporozoites differentiate and multiply producing tissue schizonts which are released releasing into the bloodstream, starting the erythrocytic cycle. Some parasites differentiate into gametocytes, enabling continued transmission. Circulating gametocytes initiate the sexual cycle in mosquitoes, culminating in sporozoite invasion of salivary glands, completing the complex life cycle across hosts and cell types.
In addition to these commonalities, the life cycle of P. vivax has unique biological features that are worth highlighting as they make this parasite the most resilient species towards elimination of malaria.
- Within the liver, vivax can differentiate into a dormant stage, called the hypnozoite, which can remain dormant for days, months or even years and which upon reactivation, causes clinical relapses.
- In the blood, vivax merozoites invade predominantly, if not exclusively, reticulocytes. This has hampered the development of a continuous in vitro culture making very challenging working with this species as parasites are only obtained from human patients or experimental infections in animal models in very low amounts.
- Circulating gametocytes appeared in peripheral blood before clinical symptoms; thus, being transmitted to mosquitos before patients seek for health care to receive antimalaria treatment.
- Last but not least, cryptic erythrocytic infections in the spleen and bone marrow, directly from merozoite invasion or via infected red blood cells, are found in these tissues during asymptomatic chronic infections. As more than 98% of the total parasite biomass is present in these erythroid tissues and they can transmit the disease to mosquitoes, this represents another major challenge to malaria elimination.
Understanding and targeting these cryptic reservoirs are critical for achieving malaria elimination goals, as parasites residing in these niches appear to evade both immune responses and conventional antimalarial treatments. Of importance, our recent research indicates that infected reticulocytes secrete extracellular vesicles, nanospheres containing parasite molecules facilitating the formation of intrasplenic niches by mediating intercellular communication. Moreover, identifying the cargo of extracellular vesicles during hypnozoite experimental infections, might discover biomarkers of these stages. Strengthening research efforts in this area is essential for developing novel strategies to combat malaria and ultimately achieve its elimination.

